In recent years, chiral o-diamine has gradually become an important building block for the synthesis of drugs, bioactive molecules, functional materials and organometallic catalysts, and is also used more as chiral organic small molecule catalysts. Over the past few decades, chemists have developed a number of methods for the synthesis of chiral o-diamines, including a series of highly efficient asymmetric catalytic reactions.
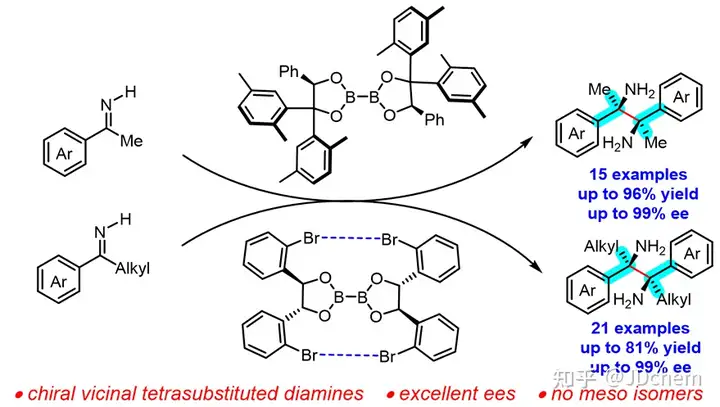
However, most of the known methods are limited to the synthesis of ortho-mono- or di-substituted chiral o-diamines, and few reports have been reported on the asymmetric synthesis of ortho-tetrasubstituted o-diamines. In order to achieve the efficient synthesis of ortho-quadripped chiral o-diamine, it is necessary to overcome many difficulties and challenges, such as steric hindrance, low reactivity of substrates, chemoselectivity, diastereoselectivity and enantioselectivity.
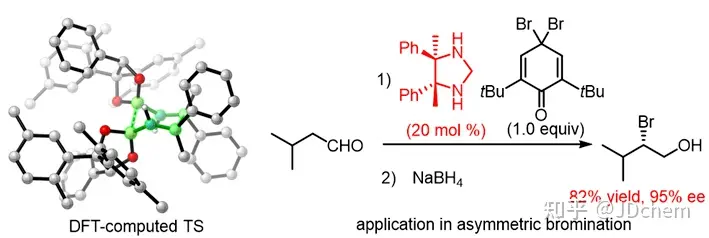
Recently, researcher Tang Wenjun and associate researcher Xu Guangqing from the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and Professor Zhong Longhua from the Department of Chemistry of Southern University of Science and Technology have cooperated to realize the asymmetric synthesis of chiral diboron-mediated ortho-tetrasubstituted o-diamine. Using a large sterically hindered chiral borate as a chiral reagent, the research team achieved the efficient reduction coupling of aryl alkyl ketone imine for the first time in a single reaction, and synthesized a series of previously unprecedentedly ortho-tetrasubstituted o-diamines, with no racemic compounds produced in the reaction. The method has the characteristics of practicality, broad spectrum, simplicity and high efficiency, and exhibits excellent diastereoselectivity and enantioselectivity. In view of the fact that different chiral borate reagents are required for different ketoimine reagents to exhibit high activity and high enantioselectivity, the research team showed that two chiral diborons adopt different conformational or assembly modes due to different non-covalent bonding (steric hindrance or Br-Br halogen bonds) in the tight transition state of the six-membered ring, effectively explaining the matching problem between different ketoimine substrates and different chiral borates. Optically pure ortho-tetrasubstituted o-diamine can be used to develop effective organic small molecule catalysts. In the asymmetric α-brominated reaction of aliphatic aldehydes, the organocatalysts derived from orthotetrasubstituted o-diamine exhibited excellent enantioselectivity.

This work is the latest breakthrough in the discovery and development of the biboron-involved [3,3]-σ rearrangement reaction, which provides an efficient, simple and practical synthesis method for ortho-tetrasubstituted chiral o-diamine and related chiral building blocks.
Tel:+86-18271874579
Mobile:+86-18971104330
E-mail: sales@JDchem.com.cn
Whatsapp: +86-18271874579

